Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 -Ga
-Ga O
O (001)
(001)Tsai, Y. H.*; Kobata, Masaaki; Fukuda, Tatsuo; Tanida, Hajime; Kobayashi, Toru; Yamashita, Yoshiyuki*
Applied Physics Letters, 124(11), p.112105_1 - 112105_5, 2024/03
Times Cited Count:0Suzuki, Tomoya*; Otsubo, Ukyo*; Ogata, Takeshi*; Shiwaku, Hideaki; Kobayashi, Toru; Yaita, Tsuyoshi; Matsuoka, Mitsuaki*; Murayama, Norihiro*; Narita, Hirokazu*
Separation and Purification Technology, 308, p.122943_1 - 122943_7, 2023/03
Times Cited Count:2 Percentile:22.61(Engineering, Chemical)HNO leaching is used in recycling Pd metal from spent products that primarily contain Ag, and most Pd residues are separated from solutions containing Ag(I). However, a small amount of Pd(II) often remains in these Ag(I) solutions. Therefore, the separation of Pd(II) and Ag(I) in HNO
leaching is used in recycling Pd metal from spent products that primarily contain Ag, and most Pd residues are separated from solutions containing Ag(I). However, a small amount of Pd(II) often remains in these Ag(I) solutions. Therefore, the separation of Pd(II) and Ag(I) in HNO solutions is essential to promote efficient Pd recycling. In this study, the separation of Pd(II) and Ag(I) in HNO
solutions is essential to promote efficient Pd recycling. In this study, the separation of Pd(II) and Ag(I) in HNO solutions was investigated using four N-donor-type adsorbents functionalized with amine (R-Amine), iminodiacetic acid (R-IDA), pyridine (R-Py), or bis-picolylamine (R-BPA). R-Amine, R-IDA, and R-Py selectively adsorbed Pd(II) over Ag(I), Cu(II), Ni(II), and Fe(III) from HNO
solutions was investigated using four N-donor-type adsorbents functionalized with amine (R-Amine), iminodiacetic acid (R-IDA), pyridine (R-Py), or bis-picolylamine (R-BPA). R-Amine, R-IDA, and R-Py selectively adsorbed Pd(II) over Ag(I), Cu(II), Ni(II), and Fe(III) from HNO solutions (0.3-7 M), but R-Amine exhibited a lower Pd adsorption efficiency. In contrast,
solutions (0.3-7 M), but R-Amine exhibited a lower Pd adsorption efficiency. In contrast,  90% of Pd(II), Ag(I), and Cu(II) were adsorbed by R-BPA over the entire range of HNO
90% of Pd(II), Ag(I), and Cu(II) were adsorbed by R-BPA over the entire range of HNO concentrations. Structural analyses of the adsorbed metal ions using Fourier transform infrared spectroscopy and extended X-ray absorption fine structure spectroscopy revealed the separation mechanisms of the N-donor-type adsorbents. Pd(II) adsorption on R-IDA, R-Py, and R-BPA occurred via Pd(II) coordination of the functional groups (iminodiacetic acid, pyridine, and bis-picolylamine, respectively), whereas that on R-Amine occurred via anion exchange of NO
concentrations. Structural analyses of the adsorbed metal ions using Fourier transform infrared spectroscopy and extended X-ray absorption fine structure spectroscopy revealed the separation mechanisms of the N-donor-type adsorbents. Pd(II) adsorption on R-IDA, R-Py, and R-BPA occurred via Pd(II) coordination of the functional groups (iminodiacetic acid, pyridine, and bis-picolylamine, respectively), whereas that on R-Amine occurred via anion exchange of NO
 with [Pd(NO
with [Pd(NO )
) ]
] . The coordinative adsorption mechanisms resulted in the higher Pd(II) adsorption behaviors of R-IDA, R-Py, and R-BPA. HCl (5.0 M) and thiourea (0.1 M) eluents desorbed 83% of Pd(II) from R-IDA and 95% from R-Py, respectively. R-Py was the most effective Pd(II) adsorbent based on adsorption selectivity and desorption efficiency.
. The coordinative adsorption mechanisms resulted in the higher Pd(II) adsorption behaviors of R-IDA, R-Py, and R-BPA. HCl (5.0 M) and thiourea (0.1 M) eluents desorbed 83% of Pd(II) from R-IDA and 95% from R-Py, respectively. R-Py was the most effective Pd(II) adsorbent based on adsorption selectivity and desorption efficiency.
 and CrUO
and CrUO
Akiyama, Daisuke*; Kusaka, Ryoji; Kumagai, Yuta; Nakada, Masami; Watanabe, Masayuki; Okamoto, Yoshihiro; Nagai, Takayuki; Sato, Nobuaki*; Kirishima, Akira*
Journal of Nuclear Materials, 568, p.153847_1 - 153847_10, 2022/09
Times Cited Count:3 Percentile:66.21(Materials Science, Multidisciplinary)FeUO , CrUO
, CrUO , and Fe
, and Fe Cr
Cr UO
UO are monouranates containing pentavalent U. Even though these compounds have similar crystal structures, their formation conditions and thermal stability are significantly different. To determine the factors causing the difference in thermal stability between FeUO
are monouranates containing pentavalent U. Even though these compounds have similar crystal structures, their formation conditions and thermal stability are significantly different. To determine the factors causing the difference in thermal stability between FeUO and CrUO
and CrUO , their crystal structures were evaluated in detail. A Raman band was observed at 700 cm
, their crystal structures were evaluated in detail. A Raman band was observed at 700 cm in all the samples. This Raman band was derived from the stretching vibration of the O-U-O axis band, indicating that Fe
in all the samples. This Raman band was derived from the stretching vibration of the O-U-O axis band, indicating that Fe Cr
Cr UO
UO was composed of a uranyl-like structure in its lattice regardless of its "x"' value. M
was composed of a uranyl-like structure in its lattice regardless of its "x"' value. M ssbauer measurements indicated that the Fe in FeUO
ssbauer measurements indicated that the Fe in FeUO and Fe
and Fe Cr
Cr UO
UO were trivalent. Furthermore, Fe
were trivalent. Furthermore, Fe Cr
Cr UO
UO lost its symmetry around Fe
lost its symmetry around Fe with increasing electron densities around Fe
with increasing electron densities around Fe , as the abundance of Cr increased. These results suggested no significant structural differences between FeUO
, as the abundance of Cr increased. These results suggested no significant structural differences between FeUO and CrUO
and CrUO . Thermogravimetric measurements for UO
. Thermogravimetric measurements for UO , FeUO
, FeUO , and CrUO
, and CrUO showed that the temperature at which FeUO
showed that the temperature at which FeUO decomposed under an oxidizing condition (approximately 800
decomposed under an oxidizing condition (approximately 800  C) was significantly lower than the temperature at which the decomposition of CrUO
C) was significantly lower than the temperature at which the decomposition of CrUO started (approximately 1250
started (approximately 1250  C). Based on these results, we concluded that the decomposition of FeUO
C). Based on these results, we concluded that the decomposition of FeUO was triggered by an "in-crystal" redox reaction, i.e., Fe
was triggered by an "in-crystal" redox reaction, i.e., Fe
 U
U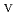
 Fe
Fe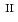
 U
U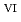 , which would not occur in the CrUO
, which would not occur in the CrUO lattice because Cr
lattice because Cr could never be reduced under the investigated condition. Finally, the existence of Cr
could never be reduced under the investigated condition. Finally, the existence of Cr in FexCr
in FexCr UO
UO effectively suppressed the decomposition of the Fe
effectively suppressed the decomposition of the Fe Cr
Cr UO
UO crystal, even at a very low Cr content.
crystal, even at a very low Cr content.
Baba, Yuji; Shimoyama, Iwao
Photon Factory Activity Report 2016, 2 Pages, 2017/00
In order to elucidate the adsorption states of radioactive Sr-90 in soil, chemical bonding states of non-radioactive strontium adsorbed on layered oxide (mica) have been investigated by X-ray photoelectron spectroscopy (XPS) and X-ray absorption near edge structure (XANES) spectroscopy. Since the number of atoms in radioactive Sr-90 is extremely small, the XPS and XANES were measured under total reflection condition of the incident X-rays. The detection limit in total reflection XPS was about 150 pg/cm , which corresponds to 300 Bq of Sr-90. The Sr 2p
, which corresponds to 300 Bq of Sr-90. The Sr 2p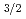 core-level energy in XPS shifted to lower energy with the decrease in the thickness of Sr layer. Also, the Sr 2p
core-level energy in XPS shifted to lower energy with the decrease in the thickness of Sr layer. Also, the Sr 2p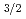
 Sr 4d
Sr 4d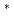 resonance energy in XANES shifts to lower energy with the decrease in the thickness. On the basis of a simple point charge model, it was elucidated that the chemical bond between Sr and mica surface becomes ionic with the decrease in the adsorbed amount of strontium.
resonance energy in XANES shifts to lower energy with the decrease in the thickness. On the basis of a simple point charge model, it was elucidated that the chemical bond between Sr and mica surface becomes ionic with the decrease in the adsorbed amount of strontium.
Nath, K. G.; Shimoyama, Iwao; Sekiguchi, Tetsuhiro; Baba, Yuji
Journal of Electron Spectroscopy and Related Phenomena, 144-147, p.323 - 326, 2005/06
Times Cited Count:5 Percentile:27.33(Spectroscopy)The effect of laser annealing on electronic atructures and molecular orientation for poly(dimethylsilane), {PDMS, [Si(CH )
) ]
] } has been studied by synchrotron radiation photoemission and photoabsorption spectroscopy. Prior to annealing, PDMS powder was mounted on the basal plane of highly oriented pyrolytic graphite. Both Si 1s X-ray photoemission spectroscopy and near-edge X-ray absorption fine structure (NEXAFS) spectroscopy at Si 1s edge show that electronic structures have been modified due to annealing. Furthermore, the angle-dependent NEXAFS spectra clearly indicate that the annealed products maintain a specific orientation. Interestingly, no such kind of orientation is present in as-received PDMS powder as no angle-dependency is observed before annealing.
} has been studied by synchrotron radiation photoemission and photoabsorption spectroscopy. Prior to annealing, PDMS powder was mounted on the basal plane of highly oriented pyrolytic graphite. Both Si 1s X-ray photoemission spectroscopy and near-edge X-ray absorption fine structure (NEXAFS) spectroscopy at Si 1s edge show that electronic structures have been modified due to annealing. Furthermore, the angle-dependent NEXAFS spectra clearly indicate that the annealed products maintain a specific orientation. Interestingly, no such kind of orientation is present in as-received PDMS powder as no angle-dependency is observed before annealing.
 hole-assisted electronic processes in the Pr
hole-assisted electronic processes in the Pr Sr
Sr MnO
MnO (x=0.0, 0.3) system
(x=0.0, 0.3) systemIbrahim, K.*; Qian, H. J.*; Wu, X.*; Abbas, M. I.*; Wang, J. O.*; Hong, C. H.*; Su, R.*; Zhong, J.*; Dong, Y. H.*; Wu, Z. Y.*; et al.
Physical Review B, 70(22), p.224433_1 - 224433_9, 2004/12
Times Cited Count:30 Percentile:75.04(Materials Science, Multidisciplinary)no abstracts in English
 and SiCl
and SiCl by resonant photoexcitation at chlorine K-edge
by resonant photoexcitation at chlorine K-edgeBaba, Yuji; Yoshii, Kenji; Sasaki, Teikichi
Surface Science, 376(1-3), p.330 - 338, 1997/00
Times Cited Count:25 Percentile:78.49(Chemistry, Physical)no abstracts in English
Yoda, Osamu; Miyashita, Atsumi; *; *; *
Excimer Lasers and Applications III, p.463 - 466, 1991/00
no abstracts in English
Miyazaki, Yasunori; Toigawa, Tomohiro; Gejo, Tatsuo*; Kumaki, Fumitoshi*; Adachi, Junichi*; Nagasaka, Masanari*
no journal, ,